top of page

myLaminin Perspectives


Breaking Down the Essentials of HIPAA Compliance
Managing and protecting health information is both a legal and ethical obligation for healthcare and research institutions. HIPAA, the Health Insurance Portability and Accountability Act, sets federal standards to safeguard Protected Health Information (PHI). It gives individuals rights over their data, restricts disclosure, and requires safeguards. Covered entities and business associates, such as research platforms, must comply through privacy, security, and breach notifica
Nashia Hussain
Aug 25, 20259 min read


Enhancing Secure Collaboration in Research: The Role of RDM Platforms
Research is increasingly collaborative, complex, and global. Whether it’s a multi‑site clinical study, a cross‑university climate project, or a public‑private health data initiative, researchers now work across institutions and nations. That kind of collaboration depends on systems—not just goodwill. Platforms like myLaminin provide secure support for research operations and data exchange via robust repositories, role‑based access, metadata standards, FAIR compliance, and aud

Keagan James
Jul 31, 20254 min read

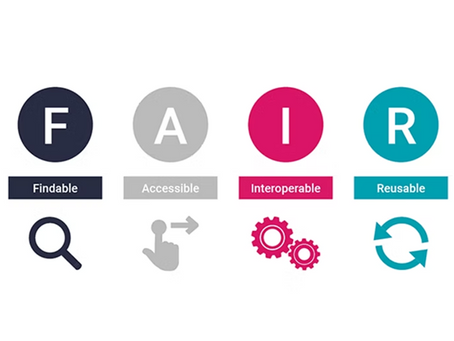
What Research Project Metadata Should be Captured to Support FAIR and Open Science Principles?
To support FAIR and Open Science principles, research projects must capture metadata across three categories: descriptive (e.g., title, keywords), administrative (e.g., funding, ethics), and technical (e.g., file formats, tools). Structured metadata makes research discoverable, accessible, ethical, and reusable—extending its value. Tools like myLaminin streamline this through integrated metadata capture and research data management.

Alain Lai
May 27, 20254 min read


Why Researchers Must Rethink Research Data Management: The Case for a Commercial RDM Solution
Higher Education Researchers must evaluate commercial options to address new Research Data Management requirements.

Ash Bassili
Apr 4, 20253 min read
bottom of page